Med Aditus Pharmaceuticals, Inc. is emerging as a leader in the pharmaceutical manufacturing sector in sub-Saharan Africa. It will be the first company to deploy Continuous Modular Pharmaceutical Manufacturing (CMPM) – an approach to manufacture pharmaceutical products that involves uninterrupted movement of materials through various stages of the manufacturing process.
Unlike traditional batch processing, CMPM operates as a continuous process: from introduction of excipients and drug substances to a high-efficiency mixer followed by compression of the powder mix into tablets as a finished drug product. This allows for a more streamlined, efficient, and flexible production process. This proven approach will ensure consistent quality, high efficiency, and faster time-to-market for life-saving medications in sub-Saharan Africa and beyond.
Traditional batch manufacturing involves multiple unit operations, spanning several days to weeks, and is not fully automated. At each step of the process, production halts to ensure quality control before resuming, contributing to a much slower production schedule in comparison to the continuous process. If a batch fails a quality test, it has to be discarded causing waste and adverse environmental impact. Because the batch process requires a long turnaround time, it is much less responsive to market demand.
With CMPM, the quality of the material is monitored continuously: Any deviation from the quality specification is identified in real-time and corrected immediately, preventing waste. CMPM’s smaller footprint requires a lower initial and subsequent capital investment. The production lines are fully automated, and the high-capacity and flexible production lines can be quickly adjusted to meet market demands or specific product requirements. CPMP is easily scalable for expanding production capacity at the same production facility or distributed over multiple sites.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
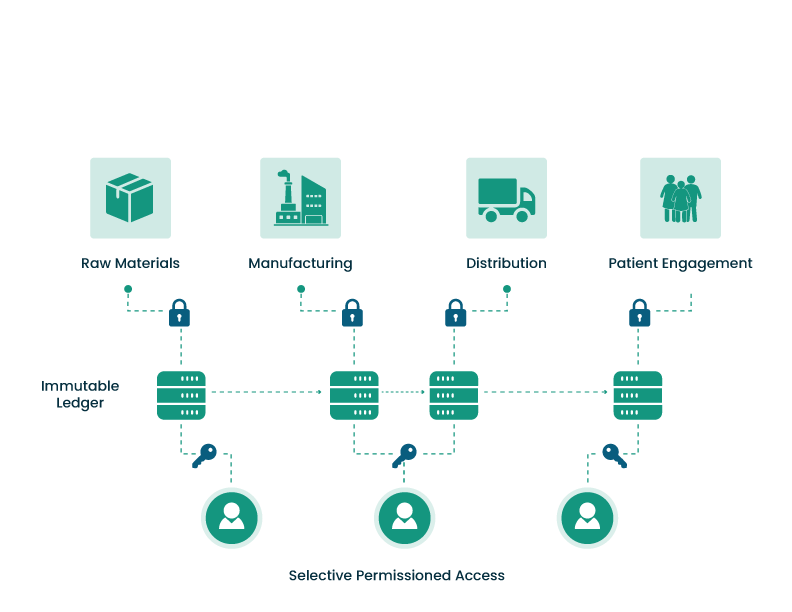
Med Aditus Pharmaceuticals, Inc. integrates blockchain-powered digital quality management architecture into the manufacturing and distribution/supply chain operations to produce and distribute high-quality pharmaceutical products at low cost in Kenya, East Africa, sub-Saharan Africa, and pan-Africa. Blockchain will make it possible to track medication adherence and outcomes securely at the point of patient consumption, utilizing smartphone and wearable devices to close the loop – manufacture, distribution, patient outcomes. Africa’s rapid growth with regards to mobile connectivity and smartphone penetration makes proactive patient engagement within reach, contributing to ongoing progress toward improving quality-of-life metrics.
Despite being one of the most highly regulated industries in the world, relatively little attention has been paid to the environmental impact of pharmaceutical manufacturing. Because of its small footprint, continuous monitoring of quality, and smart operating system, CMPM minimizes wastes, reduces energy consumption, and optimizes the use of resources.
Specifically, CMPM utilizes the implementation of in-line monitoring and control systems, enhancing process safety and product quality. Overall, continuous flow pharmaceutical manufacturing represents a sustainable approach that prioritizes environmental stewardship while delivering safe and effective pharmaceutical products to meet regional healthcare needs.